
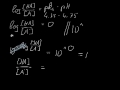

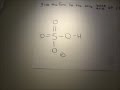






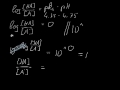






KH2PO4 is a base however the ion H2PO4- is an amphiprotic substance. What is H2PO4-? H2PO4- is called dihydrogen phosphate ion. It is the conjugate base of Phosphoric Acid H3PO4 and the conjugate... Conjugate acid and base 1 answer below » In the reaction, H2PO4- + HAsO42- HPO42- + H2AsO4-, which species are a conjugate acid-base pair? Feb 21 2012 11:05 AM The conjugate base of an acid, any acid, is defined as the acid "LESS" a proton, H^+. The conjugate acid of a base, any base, is defined as the base "PLUS" a proton. Phosphoric acid, H_3PO_4, is the parent acid. If it loses a proton, H^+, we conserve both mass and charge, and H_2PO_4^- results. And what is the conjugate base of this beasty? Again, conserve mass and charge, and HPO_4^(2-) results. You did not conserve mass and charge in your question; I agree that this is all too Conjugate Acid Of H2po4. Source(s): https://shrink.im/a0ffX. 0 0. Anonymous. 5 years ago. For the best answers, search on this site https://shorturl.im/8dmlP. congugate acid is H+ and conjugate base is PO4-0 0. Anonymous. 6 years ago. problematic subject. do a search on yahoo or google. just that can assist! 0 3. Still have questions? Get your answers by asking now. Ask Question + 100. Join The conjugate base of H 2 PO 4 - is HPO 4 -2. The removal of a proton (H +1) from a conjugate acid gives us its conjugate base. Removing a +1 charge from... See full answer below. To determine the conjugate acid of any conjugate base, simply give a hydrogen ion back to the base. H3PO4 + H2O = H3O+ + H2PO4-. So, H3PO4 is the conjugate acid of H2PO4-. Bronsted/Lowry conjugate acid/base pairs can be seen in the reversible reaction above. Answer to The formula for the conjugate base of H2PO4- is . 2. The formula for the conjugate acid of HSO3- is its an acid, H2PO4- is called dihydrogen phosphate ion. It is the conjugate base of Phosphoric Acid H3PO4 and the conjugate acid of monohydrogen phosphate ion HPO42 What is H2PO4-? H2PO4- is called... Answer. The concept of conjugate Acid-Base pair was given by Lowry-Bronsted Theory. According to the theory: Acids are proton (H +) donors. Bases are proton (H +) acceptors. Hence, in order to get the conjugate base of a species, we should consider that substance as an acid and vice versa. Thus, HPO42- is the conjugate base of H2PO4- (which is an acid in step II, but is the conjugate base of H3PO4 in step I) H3PO4 is a tribasic acid, thus ionising in three steps.The conjugate base is formed when an acid loses its proton.
[index] [6333] [2686] [6909] [8433] [4596] [9616] [3143] [7106] [7394] [390]
Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... Determine acid/base ratio of a buffer Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... Determine acid/base ratio of a buffer - Duration: 12:43. Peter Klappa 38,107 views. 12:43. Using the HH equation to calculate partial charges on amino acids - Duration: 5:26. ...
Copyright © 2024 hot.topsportnews.site